Future Directions
Research On the Genetics of Eczema
With the breakthrough that has researchers involved in the study of Eczema excited, the identification of strong correlations between mutations in the filaggrin gene and atopic dermatitis opens up many doors for future advances in the treatment and diagnosis of the condition.
With previous treatments focused mainly on subduing the irritation and redness that accompanies eczema inflicted skin, new directions focus on the more permanent and long-term remediation of the integrity of the epidermal layer. With the prevalent loss-of-function mutation observed in eczema patients, most commonly in R501X and 2284del4, a lowered production of the Filaggrin protein is the consequence correlated to the higher rate of atopic dermatitis. Increasing the concentration of this protein in the epidermis, or its expression by the gene, could help in alleviate the stress on the skin exhibited by eczema patients naturally suffering from a deficiency. (1,2)
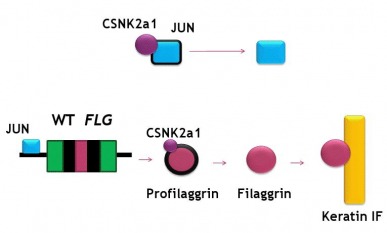
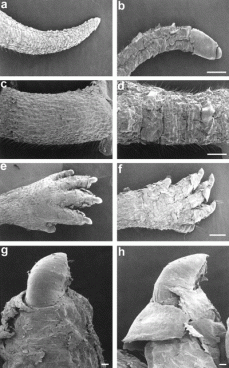
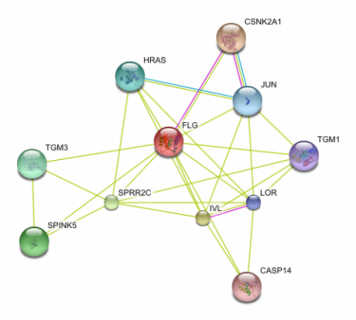
Using small molecules, I believe that we can manipulate the filaggrin pathway to help upregulate the production of the protein to get the desired result. As shown in Figure 1, a mouse model experiment showed reduced filaggrin expression in these mutants with the flaky skin characteristic of eczema. Using STRING to look at protein interactions of the filaggrin gene product, I found that a particular protein has a promising function for future research for the design of a possible treatment method. CSNK2A1, as seen in the upper right hand corner of Figure 2, encodes for Casein Kinase II. This protein is involved in the processing of a transcription factor, JUN, as well as the process of profilaggrin, the filaggrin precursor. JUN, after processing by CSNK2A1, activates the trancription of the filaggrin gene. What we can hypothesize is that in up-regulating this Casein Kinase II protein, we can both increase transcription of the filaggrin gene as well as the processing of the precursor into the important filaggrin final protein product. As seen in the predicted pathway I have outlined in Figure 3, this should help result in the increased availability of the filaggrin protein for binding to the keratin intermediate filaments. This may result in the restoration of the integrity of the epidermal layer resulting from an increase in aggregation of the keratin intermediate filaments bound by the filaggrin. (3,4,5)
With previous treatments focused mainly on subduing the irritation and redness that accompanies eczema inflicted skin, new directions focus on the more permanent and long-term remediation of the integrity of the epidermal layer. With the prevalent loss-of-function mutation observed in eczema patients, most commonly in R501X and 2284del4, a lowered production of the Filaggrin protein is the consequence correlated to the higher rate of atopic dermatitis. Increasing the concentration of this protein in the epidermis, or its expression by the gene, could help in alleviate the stress on the skin exhibited by eczema patients naturally suffering from a deficiency. (1,2)
Using small molecules, I believe that we can manipulate the filaggrin pathway to help upregulate the production of the protein to get the desired result. As shown in Figure 1, a mouse model experiment showed reduced filaggrin expression in these mutants with the flaky skin characteristic of eczema. Using STRING to look at protein interactions of the filaggrin gene product, I found that a particular protein has a promising function for future research for the design of a possible treatment method. CSNK2A1, as seen in the upper right hand corner of Figure 2, encodes for Casein Kinase II. This protein is involved in the processing of a transcription factor, JUN, as well as the process of profilaggrin, the filaggrin precursor. JUN, after processing by CSNK2A1, activates the trancription of the filaggrin gene. What we can hypothesize is that in up-regulating this Casein Kinase II protein, we can both increase transcription of the filaggrin gene as well as the processing of the precursor into the important filaggrin final protein product. As seen in the predicted pathway I have outlined in Figure 3, this should help result in the increased availability of the filaggrin protein for binding to the keratin intermediate filaments. This may result in the restoration of the integrity of the epidermal layer resulting from an increase in aggregation of the keratin intermediate filaments bound by the filaggrin. (3,4,5)
The implications by research surrounding small molecule interference in the filaggrin pathways are potentially bringing creams or oral supplements to the market that contain these suggested proteins, such as CSNK2a1. These molecules would perturb the pathway on the skin in a way that increases the expression of proteins required for normal epidermal function. Another STRING could be performed on proteins such as CSNK2a1 to establish other activating proteins to accompany the Casein Kinase II in the medication ingredients to maximize the affect on the filaggrin pathway. The aims would be to ultimately restore the normal phenotype in eczema patients for a more prolonged and permanent solution than currently offered by topical or oral treatments.
.
References
1. Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. (2006). "Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis". Nat. Genet. 38 (4): 441–6.
2. Smith FJ, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38(3):337–342.
3. Presland RB, Boggess D, Lewis SP, Hull C, Fleckman P, Sundberg JP. Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J Invest Dermatol. 2000;115(6):1072–1081.
4. STRING http://string-db.org/
5. Haugen-Scofield, J., Resing, K.A., and Dale, B.A. (1988). Characterization of an epidermal phosphatase specific for filaggrin phosphorylated by casein kinase II. J. Invest. Dermatol. 91, 553-559.
1. Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. (2006). "Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis". Nat. Genet. 38 (4): 441–6.
2. Smith FJ, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38(3):337–342.
3. Presland RB, Boggess D, Lewis SP, Hull C, Fleckman P, Sundberg JP. Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J Invest Dermatol. 2000;115(6):1072–1081.
4. STRING http://string-db.org/
5. Haugen-Scofield, J., Resing, K.A., and Dale, B.A. (1988). Characterization of an epidermal phosphatase specific for filaggrin phosphorylated by casein kinase II. J. Invest. Dermatol. 91, 553-559.